[In 2015] the world said hello to Homo naledi, a new species of ancient human discovered in South Africa’s Rising Star cave… at least 15 individual skeletons—one of the richest hauls of hominid fossils ever uncovered.
But one significant problem clouded the excitement over the discovery: The team doesn’t know how old the fossils are…Everyone from professional paleontologists to interested members of the public raised the same question: Why hadn’t the team dated the fossils yet?
The simple answer is: Because dating fossils is really difficult.
Scientific papers and news reports about new fossils so regularly come with estimates of age. I asked John Hawks, a biologist at the University of Wisconsin and one of the heads of the Rising Star expedition, to talk me through the various available methods—and why they have been difficult to apply to the latest finds.
The technique people are most likely to have heard of is carbon dating.
All living things contain carbon which is the primary component of proteins, lipids, nucleic acids, and carbohydrates.
Carbon is an element composed of protons and neutrons, located in the atomic nucleus, plus the electrons on the outside of the nucleus. 
Each element is defined by its weight and electrical potential provided by
- the mass in the number of neutrally charged neutrons
- plus the mass in the positively charged protons
- which are electrically neutralized by the negatively charged electrons,.
Shared electrons is what bonds molecules into compounds such as C02, carbon dioxide.
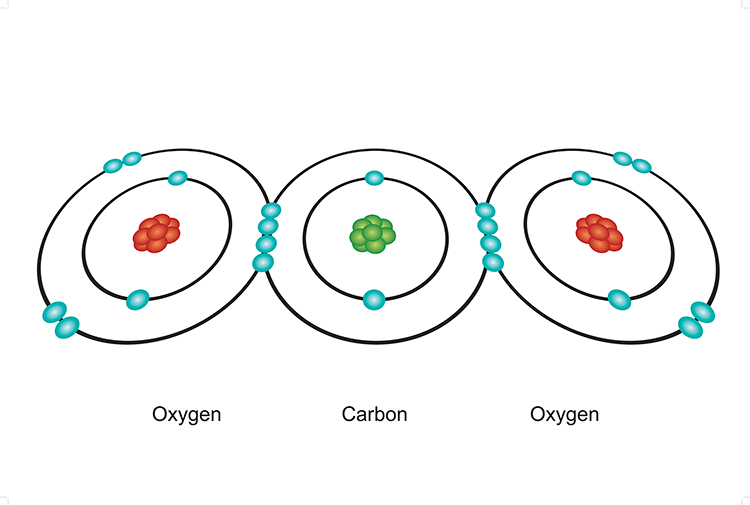
The balance between the number of protons and electrons determining the electrical potential of the atom / ability to bond into large compounds is what matters most. The number of neutrons can vary, changing the mass of but remaining the same element, identified as isotopes of that element.
For example, the element carbon has six protons, but can have six, seven, or eight neutrons. Thus, carbon has three isotopes: carbon 12 (12C), carbon 13 (13C), and carbon 14 (14C).
Some isotopes have an “unstable” nucleus, meaning that it will change its number of protons, neutrons, or both. This change is called radioactive decay. For example, unstable 14C transforms to stable nitrogen (14N).
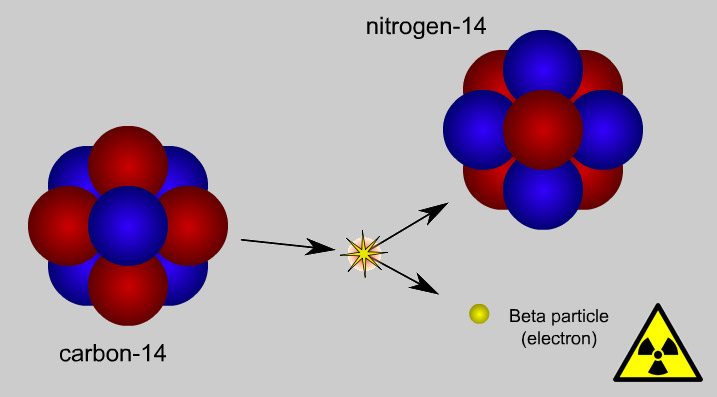
The atomic nucleus that decays is called the parent isotope. The product of the decay is called the daughter isotope. In the example, 14C is the parent and 14N is the daughter.
The amount of time that it takes for half of the parent isotope to decay into daughter isotopes is called the half-life of an isotope…If the half life of an isotope is known, the abundance of the parent and daughter isotopes can be measured and the amount of time that has elapsed since the “radiometric clock” started can be calculated.
“Some of the lighter isotopes were formed very early in the history of the universe, during the Big Bang.”
Press pause. The Big Bang is an idea that has lost considerable ground in astronomy the more research o has been performed, see the relevant post for details.
“Others result from processes that happen within stars or as a result of chance collisions between highly energetic nuclei – known as cosmic rays – within our atmosphere.”
Whoa! This cannot be stated as a known fact. What is a recently established fact is that the Van Allen radiation belt above earth’s atmosphere is a zone of energetic charged particles held around a earth by its magnetosphere. “Scientific” bias is evident and must be taken into consideration in all its claims.
Isotopes are unstable, however, and over time decay into an atomic structure with a matched set of protons and neutrons.
“The rate of decay for many radioactive isotopes has been measured and does not change over time.”
Whoa! Science cannot claim that the rate of decay of radioactive isotopes formed at The Big Bang haven’t changed over the billions of years claimed to have passed since that claimed event! We don’t need to be scientifically trained to read with critical thinking skills, especially when the inconsistencies are so apparent leading inevitably to a foregone conclusion.
Thus, radioactive isotopes ticking along regularly like a clock provide estimates of the age of certain geological materials, i.e. certain rocks, associated with fossils, or the fossil material itself.
Whoa again! “Associated with fossils” does not prove a perpetual partnership!
And there is more to what becomes more and more evident is a shell game.
But there is significant uncertainty in carbon dating.
- First…the proportions of C-14 in the atmosphere in historic times is unknown….the atmospheric ratio is known to vary over time…
- various plants have differing abilities to exclude significant proportions of the C-14 in their intake. This varies with environmental conditions as well…An animal that ingested plants with relatively low C-14 proportions would be dated older than their true age.
The method is far less predictive of age than is commonly supposed, especially for older samples.
Whoa! So what methods are used to establish dates older than 50,000 years ago, i.e. millions of years on the calendar of the evolution of all living things?
An alternative technique, known as electron spin resonance or ESR…is great for dating teeth…in a way that depends on two things: the levels of natural radiation in its environment, and how long it was buried for.
If you know the former, you can deduce the latter.
But knowing the natural radiation levels is “sort of nightmarish,” says Hawks. It involves, for example, installing actual radiation dosimeters and taking out vertical cores of sediment. And even then, the results from ESR typically need to be cross-checked against other sources of data.
The same problem as with radiocarbon-14 – no certain knowledge of the amount of carbon-14 in the atmosphere or organism at the point of death.
Radioactive carbon-14…used to analyze an organic material, such as wood, seeds, or bones, to determine a date of the material’s growth…need to be calibrated with other dating techniques to ensure accuracy…
Dendrochronology, founded in the late 1800s and early 1900s, allowed scientists to mark exact calendar dates for each ring…
Long tree-ring sequences have been developed throughout the world...An extensive tree-ring sequence from the present to 6700 BC was developed in Arizona using California bristlecone pine, some of which are 4900 years old, making them the oldest living things on earth.
This provides scientific validation of the biblical model of earth’s living history beginning about 6,000 years ago, with Noah’s flood approximately 1,000 years later.
Paleontologists can sometimes date a new fossil by looking at its companions in death, by finding nearby bones of other extinct animals that died within a known timeframe.
As a defense attorney, I would “Strike that!”
- Being in the vicinity does not prove being accomplices or companions in death.
- What is the proof for the ‘known” timeframe of the death of the other animals?
There is a much bigger issue that has been skirted in the above discussion about dating fossils. Fossils contain no organic remains suitable for dating with the carbon-14 or magnetic spin resonance methods. So why are the “experts” even bringing these methods into the discussion?
Smokescreen for the lack of any dating methods at all?
Fossils are the preserved…traces…of ancient organisms.
Fossils are not the remains of the organism itself! They are rocks.
Fossils are formed…when a living organism…is quickly buried
- by sediment from ground water carrying dissolved minerals which fill up space inside
- the cells of plants and animals leaving petrified body parts, such as a bone, or
- the shape of a body part or plant that is then entirely removed by ground water flow
- or ash from a volcanic eruption.
Fossils are therefore either sedimentary or volcanic / igneous (fiery as in “ignite”).
And of the two, only igneous rocks can be dated.
The Rift Valley is the result of an ancient series of faults, rifts, and volcanoes deriving from the shifting of tectonic plates at the junction between the Somalian and the African plates…
The faults occurred in…a time span of some 500 million years.
They forge on. (Haha, get the pun?)
The volcanic material in tuff is well-suited for radiometric dating [via] an unstable isotope of potassium.
Or not.
3.2.2.1 Problems of 40K/40Ar Dating
The fundamental assumptions in potassium-argon dating are that
(a) no argon was left in the volcanic material after formation and
(b) the system has remained closed since the material was produced, so that no argon has either entered or left the sample since formation.
The former assumption may be invalid in the case of…previously formed argon during formation under high hydrostatic pressure.
Once again evidence of massive flooding.
Similarly, certain rocks may have incorporated older “argon-rich” material during formation.
Such factors result in the sample age being overestimated (Fitch, 1972)…
A lava flow that is known to have taken place in 1800-1801…was dated by potassium-argon as being 2,960 million years old.
The newly-formed lava dome at Mount St. Helens was dated to an age of 340,000 years when it was only 10 years old.
The K-Ar test is not trustworthy. Despite this it is still being used because it supports the preconceptions of long earth age required for Evolution.
Seriously? What is being claimed here is that
- igneous / volcanic rocks formed from material deep underground and spewed above ground
- can be used to assign the same date to the formation of above-ground sedimentary rock layers whose material has always been exposed to the atmospheric elements and which were formed by water-born events either slowly over time or during a rapid catastrophic flood event
- because they were thrown together during tectonic events that shook the earth hard enough to split it wide open.
This is clearly not scientific.

